NH3 ammine H2O aqua or aquo CO carbonyl NO nitrosyl. The ions in an ionic compound stack up in a regular repeating pattern called a ____ in order to maximize attraction and minimize repulsion White Most ionic compounds that do not contain transition metals are what color.
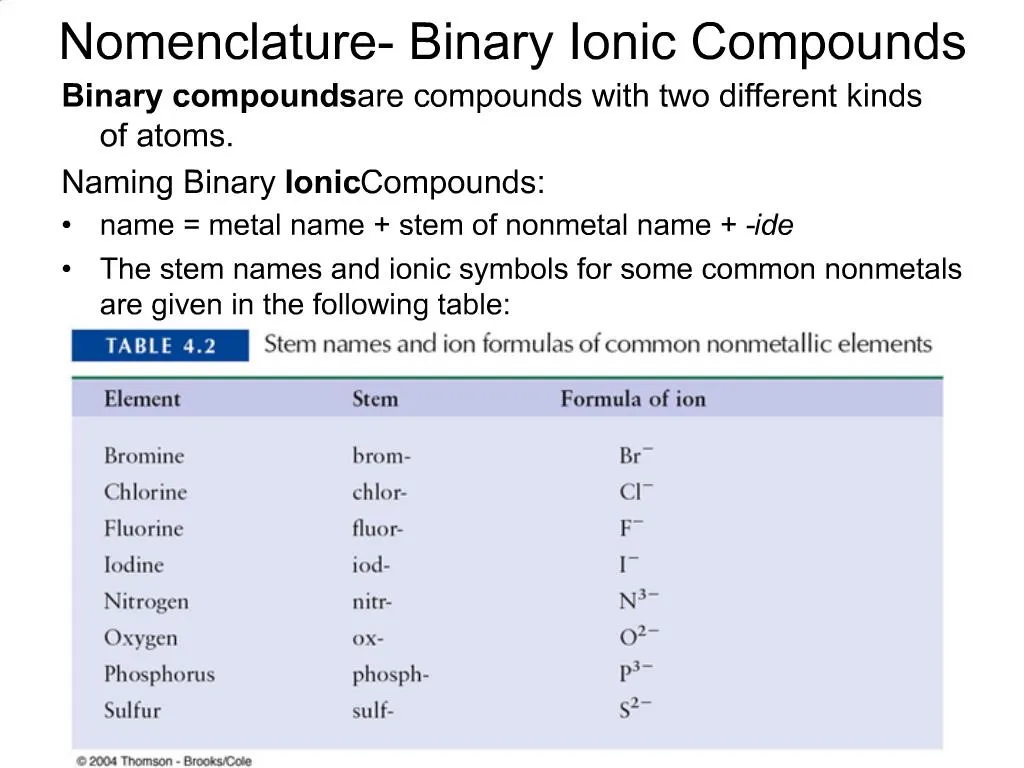
Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132
Nomenclature of common acids.
. Simple acids known as binary acids have only one anion and one hydrogen. The compounds named via trivial nomenclature often have much shorter and simpler names than the corresponding IUPAC nomenclature of the same compounds. An example of this relative ease of naming compounds can be seen in the following example A type of carboxylic acid which is generally found in tamarind is referred.
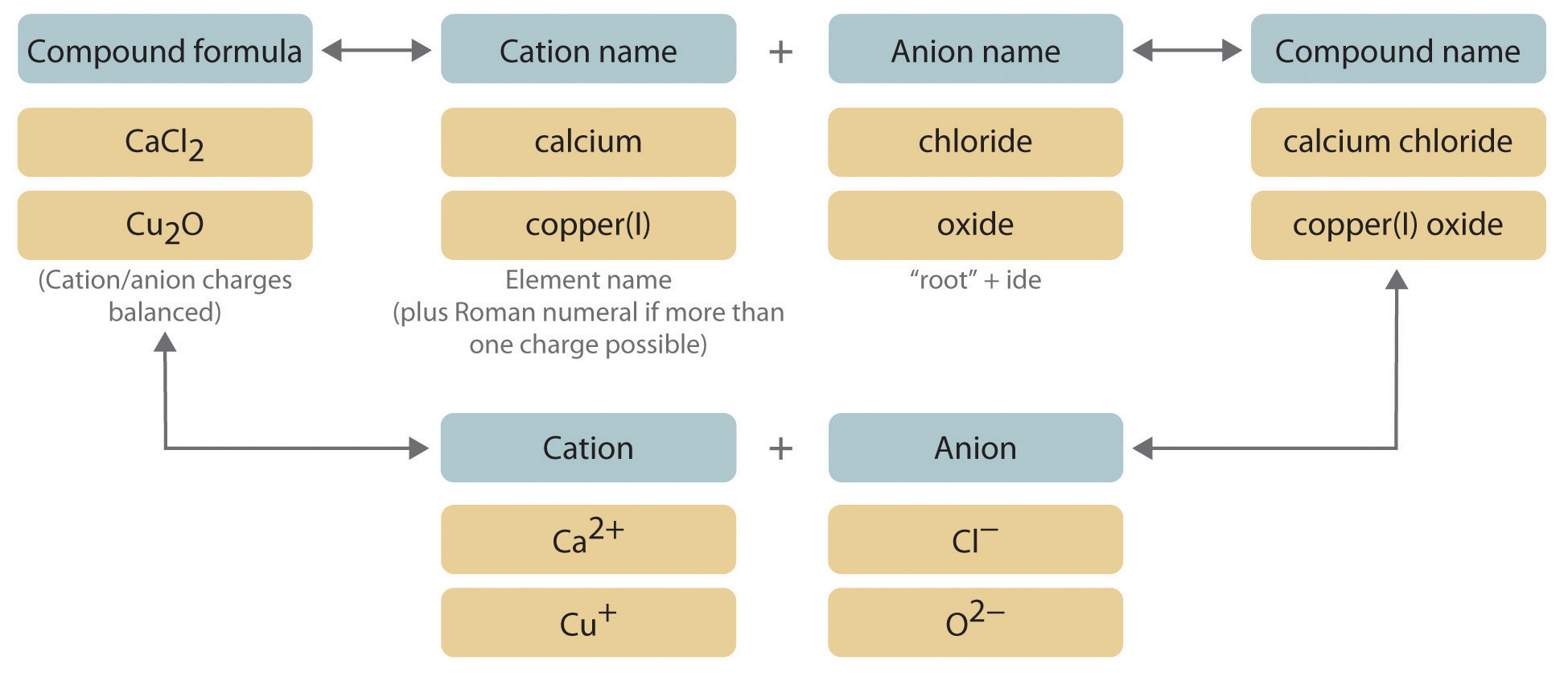
For this reason this system is still not obsolete today. After the ligands are named the name of the central metal atom is written. These anions usually have the ending -ide As acids these compounds are named starting with the prefix hydro- then adding the first syllable of the anion then the suffix -ic For example HCl which is hydrogen and chlorine is called hydrochloric acid.
The following neutral ligands are assigned specific names in coordination compounds. If the complex has an anionic charge associated with it the suffix -ate is applied.
4 5 Formulas And Names Of Binary Ionic Compounds Chemistry Libretexts

Forming And Naming Ionic Compounds Type 1 And 2 Binary Compounds Ppt Download

Naming Binary Ionic Compounds Rules Examples Expii

Solved Part Ii Binary Ionic Compounds Containing Chegg Com

Naming Ionic Compounds A Guided Inquiry Exercise


0 comments
Post a Comment